

 JULIAN LILLEY explains which glue to use in which situation.
JULIAN LILLEY explains which glue to use in which situation.
Glues have their origins dating back to as long ago as 4000 BC. They came into being when ancient tribes discovered that a sticky material (collagen) could be extracted from bones, hides and skin. These types of starting materials along with other animal scraps were processed to produce a glue liquor which was thickened and maybe other chemicals added. With these same basic processes bone glue, hide or skin glue and fish glue are produced.
Plants have also been used to produce vegetable glue such as gum Arabic from the acacia.
Archaeologists excavating burial sites from 4000 BC have discovered clay pots repaired with glue made from tree sap. Ancient Greeks developed adhesives for use in carpentry, and created recipes for glue from egg whites, blood, bones, milk, cheese, vegetables and grains. The Romans used tar and beeswax for glue. Around 1750, the first glue or adhesive patent was issued in Britain. The glue was made from fish. Patents were then rapidly issued for adhesives using natural rubber, animal bones, fish and starch.
So the term adhesive is a general term which includes products formulated from polymers produced in a modern chemical plant. These are often called synthetic "resins", so named after the gooey substance found in pine trees, which was one of the first widely used adhesives. Glues may also be, as we have seen, natural adhesive products made from animal parts.
The definition of an adhesive is "any substance applied to the surfaces of materials that binds them together and resists separation." So how do they work?
Essentially, like many things in the world, it is all about forces. The two key forces at work in a joint between two surfaces are both adhesive and cohesive.
Let's say you want to stick together two bits of wood, A and B, with an adhesive called C. You need three different forces here: adhesive forces to hold A to C, adhesive forces to stick C to B, and cohesive forces to hold the adhesive, C together.

If that's not obvious, think about sticking a station master’s hat to the ceiling! The glue clearly has to stick both to the hat and to the ceiling. But if the glue itself is weak, it doesn't matter how well it sticks to the hat or the ceiling because it will simply break apart in the middle.
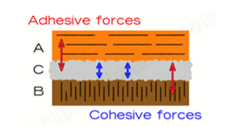
Fig 1.
These come from 3 main areas:
The adhesive forces at the surface are generated in a similar way, and come from two sources: a) within the adhesive molecule itself (Fig 1 red arrows) and between the adhesive and the surface to which it is applied.
In addition, we have the concepts of:
Here the adhesive must "wet" the surface to which it is applied.

Fig 2.
Again weak electrostatic forces between the glue molecules and the molecules on the surface come into play. For adhesives to work well like this, they have to spread and wet the surfaces very well. If the adhesive beads up, it means that the adhesive has a greater affinity for itself rather than the surface to which it is being bonded.
To explain the concept of surface energy, this is a measure of the attraction that individual molecules (e.g. of glue) have for other molecules and also for themselves. So, if the glue molecules have a low surface energy they will have a higher attraction to the molecules on the surface being bonded than to themselves, they will spread onto the surface forming a strong glue joint. Conversely, if the molecules have a higher surface energy than the surface, they are more attractive to themselves and will bead up and remain as a droplet. The surface energy or "bond ability" of various materials is shown below.
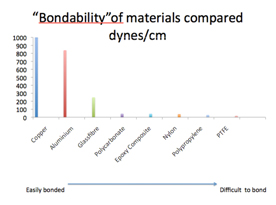
With physical bonding, there's no actual chemical bond between the adhesive and the surface it's sticking to, just a huge number of tiny attractive forces that also carry it into the microscopic voids thus increasing the adhesive force. This process is called adsorption. The analogy I would use is that the glue can seep into those voids and grip, like a climber's fingers anchoring into holes in a rock face.
In some cases, adhesives can make much stronger chemical bonds with the materials they touch through a very strong chemical connection — they effectively form a new chemical compound at the join. That process is called chemisorption. Epoxy glues have many reactive sites where strong bonds can form with high-energy metals, glasses, ceramics etc. The combination of great adhesive and cohesive strength makes epoxy an exceptionally strong adhesive specially where there are gaps to fill.
That’s enough science for now!
You may remember from a Right Lines interview in August that we are very fortunate to have in our hobby John Bristow, who is an expert chemist and also a very keen and enthusiastic modeller. He has developed a wide range of glues specifically for our wonderful hobby.
Below you will find a very useful guide as to the best glue from the Deluxe Materials range use for the job you are working on. I constantly refer to it on the copied chart I have pinned on my workshop wall (click for a larger version).
Below I have listed glue products that have been specifically designed for our hobby, all of which proved incredibly popular, in fact they are all in our top 20 best selling products.
CLICK HERE to view the current Deluxe Materials catalogue.